88888hine Health — Organic Dietary Fiber Powder & Formulation Solutions
666Shandong Shine Health Co., Ltd 2manufactures high‑qual ity organic dietary fiber powders and pharmaceutical‑grade excipients designed for food, beverage and nutraceutical formulators. Our core portfolio emphasizes soluble, low‑calorie fiber solutions — led by resistant dextrin — complemented by polydextrose, oligosaccharides (XOS/FOS/IMO) and excipients such as microcrystalline cellulose (MCC) and magnesium stearate. For samples or technical support, contact our technical sales team (info@sdshinehealth.com | WhatsApp: +86‑13405443339) .
Product highlights
- Primary ingredient: Resistant dextrin produced from non‑GMO corn starch (organic grades available).
- Key functional benefits: soluble prebiotic fiber, low‑calorie bulking, mouthfeel enhancement, and clarity compatibility for ready‑to‑drink (RTD) beverages.
- Typical specifications: Dietary fiber ≥82% (total fiber on dry basis ≥90%), solubility ≈70%, white to light yellow powder, low water activity for extended shelf life.
Core SKUs & applications
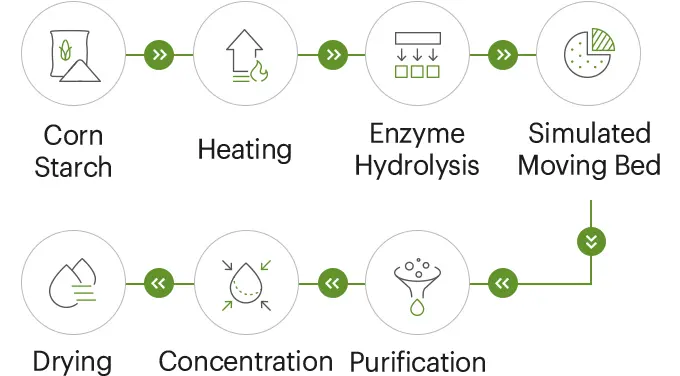
Resistant Dextrin (organic options)
Resistant dextrin is a soluble, dextrinized starch designed to resist digestion in the small intestine and act as fermentable substrate in the colon. It provides clean mouthfeel, contributes to satiety and supports steady post‑prandial glycemic response. Common applications include clear RTD beverages (1–2% to preserve clarity), yogurts, nutrition bars, bakery fortification and fiber supplements. Product page: https://www.sdshinehealth.com/resistant-dextrin/
Polydextrose & Prebiotic Oligosaccharides (XOS/FOS/IMO)
These oligosaccharides act as low‑calorie bulking agents and selective substrates for beneficial gut microbiota. They are typically used in bars, dairy, powdered drink mixes and synbiotic formulations to improve texture and deliver prebiotic functionality. Product page: https://www.sdshinehealth.com/polydextrose/
Excipients — MCC & Magnesium Stearate
Microcrystalline cellulose is supplied in multiple grades/particle sizes for tablet compression and texture control; magnesium stearate is available as a pharmaceutical lubricant tailored to formulation requirements. Datasheet: https://www.sdshinehealth.com/microcrystalline/
Technical & formulation guidance
- Recommended inclusion ranges: beverages (clear RTD, juices) 1–5% (start 1–2% for clarity); bars & snacks 3–8% for texture and fiber claims; MCC 50–90% as tablet filler depending on binder.
- Compatibility: resistant dextrin is stable across most beverage pH ranges; confirm behavior in extreme acid systems. Heat tolerance is generally good but prolonged high temperatures may change viscosity and solubility—validate in finished processing.
- Handling: pre‑dissolve under agitation for homogeneous dispersion; apply stepwise addition in high‑solids systems to prevent lumping.
- Storage & packaging: low water activity extends shelf life; recommended packaging includes 25 kg kraft bags, foil‑lined sacks and bulk containers with moisture barrier and anti‑caking protection.
Quality, certifications & supply chain
Shine Health uses non‑GMO corn starch (organic available), operates German precision production lines and imported enzymatic systems, and maintains an in‑house QC laboratory for fiber, moisture, microbiology and contaminant testing. We adhere to GMP frameworks and support export documentation for USA and EU markets. Lead times, MOQs and certification copies are available on request.
Short case studies
- Beverage clarity: a 2% resistant dextrin / 0.5% polydextrose blend delivered a clear RTD appearance, subtle mouthfeel enhancement and ~2 g added soluble fiber per 100 mL.
- High‑fiber bar: 5–7% resistant dextrin with polydextrose improved chewiness and reduced stickiness while increasing declared fiber content without adding sweetness.
Contact, samples & development support
For technical datasheets, tailored formulation trials, pilot runs or bulk inquiries, email info@sdshinehealth.com or message via WhatsApp +86‑13405443339. Our R&D team provides formulation guidance and 24/7 online technical assistance for product development and scale‑up.
FAQ (key points)
- Raw material: non‑GMO corn starch; organic grades available.
- Fiber & solubility: dietary fiber ≥82%; total fiber (dry basis) ≥90%; solubility ≈70%.
- Typical beverage inclusion: 1–5% (start 1–2% for clarity).
References
Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013.
Anderson JW, Baird P, Davis RH Jr., et al. Health benefits of dietary fiber. Nutrition Reviews. 2009.
Hasjim J., Ai Y., et al. Dextrinization and resistant starch applications. In: Advances in Food Science. 2013.
Lambeau KV, McRorie JW Jr. Fiber supplements and clinically proven health benefits. J Am Assoc Nurse Pract. 2017.
Tsitko I., Wiik‑Miettinen F., et al. In vitro fermentation models for fiber preparations. Int J Mol Sci. 2019.
Shandong Shine Health Co., Ltd. Product pages and technical datasheets. https://www.sdshinehealth.com/
Roquette / Nutriose technical literature and commercial product notes.